OXERVATE’s Mechanism and Market Impact in Sjögren’s Syndrome Care
This article explores OXERVATE, an emerging promising therapy for patients battling Sjögren’s Syndrome, a chronic autoimmune disorder that severely compromises the body’s moisture-producing glands.
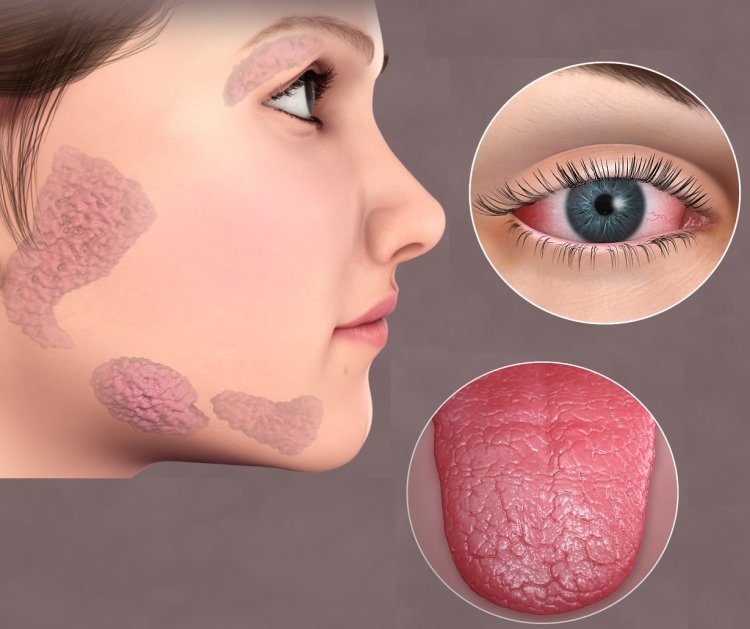
Introduction
Sjögren’s Syndrome is a chronic autoimmune disorder that primarily affects the body’s moisture-producing glands, leading to significant dryness in the eyes and mouth. This condition not only causes discomfort and visual complications but also increases the risk of corneal damage—a serious concern for many patients. Traditional treatments such as artificial tears, anti-inflammatory eye drops, and immunosuppressive therapies have provided symptomatic relief, yet they do little to address the underlying causes of the condition. Recently, the pharmaceutical landscape has witnessed a surge in innovative approaches, and one potential breakthrough is OXERVATE. Originally approved for neurotrophic keratitis, OXERVATE is now being explored for its applicability in Sjögren’s Syndrome care. With its unique mechanism of action, OXERVATE offers a fresh perspective in ocular therapeutics, promising not only symptomatic relief but also a long-term solution for nerve regeneration and corneal repair.
For more in-depth insights on OXERVATE’s development and future potential, download the full report @ OXERVATE Market Report.
Understanding OXERVATE's Mechanism of Action (MOA)
At the heart of OXERVATE’s potential lies its active ingredient—cenegermin, a recombinant form of human nerve growth factor (NGF). This OXERVATE active ingredient plays a pivotal role in maintaining corneal integrity by stimulating the survival, proliferation, and differentiation of corneal epithelial cells. OXERVATE’s Mechanism of Action is centered around its ability to promote nerve regeneration and enhance epithelial healing. In conditions such as Sjögren’s Syndrome, where chronic dry eye is prevalent and corneal health is compromised, this regenerative process is crucial.
By leveraging the neuroprotective properties of NGF, OXERVATE not only helps repair damaged corneal nerves but also supports increased tear production, addressing both the symptoms and some of the root causes of dry eye. This dual-action approach marks a significant shift from traditional therapies that merely offer temporary relief. The promise of nerve regeneration, as demonstrated by OXERVATE’s MOA, paves the way for potentially improved long-term outcomes for patients suffering from severe ocular surface disease.
For more detailed insights and the latest updates on OXERVATE, visit the OXERVATE Market update.
The Need for Innovative Treatments in Sjögren’s Syndrome
Despite the availability of symptomatic treatments for Sjögren’s Syndrome-related dry eye, there remains an urgent need for therapies that target the underlying pathophysiology of the disease. Current treatments primarily focus on alleviating discomfort without addressing the nerve dysfunction that contributes to severe ocular complications. Patients often find themselves caught in a cycle of recurring symptoms with minimal improvement in the overall health of the cornea.
Innovative treatments like OXERVATE are crucial because they offer a novel therapeutic approach that goes beyond merely treating the symptoms. The ability of OXERVATE to stimulate nerve repair and promote corneal healing directly targets the deficiencies seen in Sjögren’s Syndrome. This focus on regeneration rather than suppression could be a game changer in managing the long-term consequences of the disease. As researchers and clinicians seek more durable and effective treatments, therapies that harness the body’s natural healing processes are receiving increasing attention.
For further insights and detailed research on this breakthrough treatment, visit OXERVATE insights.
OXERVATE Sales and Market Performance
Since its initial FDA approval for the treatment of neurotrophic keratitis, OXERVATE has made impressive strides in the ophthalmic market. The sales performance of OXERVATE has been robust, reflecting a growing demand for advanced ocular treatments. Industry analysts have noted that OXERVATE sales have been on an upward trajectory, supported by its novel approach and proven efficacy in repairing corneal damage. This success has not only established OXERVATE as a trusted option for severe corneal diseases but also set a promising precedent for its future use in treating Sjögren’s Syndrome.
The remarkable progress in OXERVATE sales underscores the market’s readiness for innovative treatments that offer regenerative benefits. As pharmaceutical companies and healthcare providers consider expanding the therapeutic indications of OXERVATE, the emphasis on its unique MOA and active ingredient becomes even more significant. With continued research and positive clinical outcomes, OXERVATE sales are expected to grow further, thereby strengthening its position in the ophthalmic therapeutic landscape. Analysts believe that the current momentum in OXERVATE sales could pave the way for expanded approvals and broader market acceptance, especially if ongoing studies confirm its efficacy in treating Sjögren’s Syndrome-related dry eye.
The strong performance in OXERVATE sales serves as a testament to both its clinical potential and its market impact. Industry stakeholders are closely monitoring its progress, anticipating that successful clinical trials in Sjögren’s Syndrome could trigger another surge in OXERVATE sales, further reinforcing its market viability. The integration of robust sales figures with emerging clinical data could make OXERVATE a flagship treatment in the future, addressing an unmet need in ocular care.
Cost Considerations: OXERVATE Price and Accessibility
While the clinical benefits of OXERVATE are promising, its high cost remains a significant barrier for many patients. As a biologic therapy, the production process for recombinant human nerve growth factor is complex and subject to rigorous regulatory standards, which contribute to its steep price tag. In the United States, an eight-week treatment course with OXERVATE is reported to cost approximately $96,000. This high cost can hinder widespread adoption, especially among patients without comprehensive insurance coverage or those facing limited reimbursement options.
Efforts to address these cost concerns are critical to ensure that patients who could benefit from OXERVATE are not excluded due to financial constraints. The role of insurance companies, reimbursement programs, and patient assistance initiatives becomes paramount in bridging the affordability gap. If OXERVATE receives approval for treating Sjögren’s Syndrome, strategies to negotiate pricing adjustments based on broader indications will be essential. Such measures could not only improve accessibility but also enhance the overall market acceptance of this innovative therapy.
Moreover, the high price point of OXERVATE underscores the need for continued dialogue between healthcare policymakers and pharmaceutical companies. Collaborative efforts to make the treatment more affordable could result in expanded insurance coverage and better patient support programs. Ensuring that the benefits of OXERVATE reach a wider patient base will be crucial for its long-term success and its ability to reshape the treatment paradigm for ocular diseases.
For additional insights on OXERVATE’s transformative potential, please download the full OXERVATE report.
Clinical Trials and Future Prospects
The use of OXERVATE in treating Sjögren’s Syndrome is still under investigation, and ongoing clinical trials are pivotal in establishing its safety and efficacy for this new indication. These OXERVATE Clinical Trials are designed to evaluate whether the regenerative properties of NGF can significantly improve the ocular surface health of patients suffering from Sjögren’s Syndrome. Early findings from these trials are highly anticipated, as they will inform the potential expansion of OXERVATE’s indications beyond neurotrophic keratitis.
Future prospects for OXERVATE hinge on the outcomes of these clinical trials and the subsequent regulatory review process. Positive trial results could lead to additional OXERVATE Approvals, enabling the treatment to be used more widely in managing Sjögren’s Syndrome-related dry eye. The potential for regulatory expansion not only signifies a major therapeutic advancement but also offers hope to a patient population that has long struggled with inadequate treatment options.
As clinical data continue to accumulate, stakeholders are eager to see if OXERVATE can replicate its success in neurotrophic keratitis within the realm of Sjögren’s Syndrome care. The possibility of improved corneal healing, increased tear production, and overall better ocular health could redefine the treatment landscape for this challenging autoimmune disorder. Moreover, the successful integration of OXERVATE into Sjögren’s Syndrome treatment protocols would likely stimulate further investments in research and development, potentially catalyzing a new era of regenerative ophthalmology.
With its promising mechanism of action and positive market performance, OXERVATE stands at the forefront of innovation in ocular therapeutics. The next few years will be crucial as additional data emerge, and the healthcare community evaluates the long-term benefits of this groundbreaking treatment. Whether through additional clinical trials or expanded regulatory approvals, the future for OXERVATE in Sjögren’s Syndrome care appears bright.
For those looking to explore more about this breakthrough treatment, download the full OXERVATE Insights Report.
Conclusion
OXERVATE represents a significant potential advancement in the management of Sjögren’s Syndrome-related dry eye. With its unique active ingredient and regenerative mechanism of action, it offers a promising alternative to traditional symptomatic treatments. By addressing the underlying nerve dysfunction that contributes to severe ocular complications, OXERVATE has the potential to provide long-lasting benefits and improved quality of life for patients.
Despite its high cost, the strong performance in OXERVATE sales demonstrates the market’s readiness to embrace innovative therapies. Continued research through clinical trials will be critical in confirming its efficacy and paving the way for expanded indications and approvals. As the therapeutic landscape evolves, the integration of novel treatments like OXERVATE could reshape how Sjögren’s Syndrome and other ocular conditions are managed.
The compelling combination of cutting-edge science and market potential makes OXERVATE a beacon of hope in the realm of regenerative ophthalmology. For patients, clinicians, and industry stakeholders alike, the journey toward improved ocular health is set to take a transformative leap forward with the advancement of OXERVATE.
Related Reports
- Dry Eye Disease Market Insight, Epidemiology And Market Forecast Report
- Sjogren's Syndrome Market Insight, Epidemiology And Market Forecast Report
- Ocular Hypertension Market Insight, Epidemiology And Market Forecast Report
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.
What's Your Reaction?















